2020 China New Design Sars-Cov-2 Antigen - Dual Biosafety System Device for SARS-CoV-2 Antigen Rapid Test – Liming Bio
2020 China New Design Sars-Cov-2 Antigen - Dual Biosafety System Device for SARS-CoV-2 Antigen Rapid Test – Liming Bio Detail:
TheStrongStep® SARS-CoV-2 Antigen Rapid Test is a rapid immunochromatographic assay for the detection of COVID-19 antigen to SARS-CoV-2 virus in human Throat/Nasopharyngeal swab. The assay is used as an aid in the diagnosis of COVID-19.
IMPORTANT: THIS PRODUCT IS INTENDED FOR PROFESSIONAL USE ONLY, NOT FOR SELF-TESTING OR TESTING AT HOME !
For use by clinical laboratories or healthcare workers only
For Medical Professional Use Only
For test Midstream
Bring the kit components to room temperature before testing. Open the pouch and remove the test device.
Once opened, the test device must be used immediately.
Label the test device with patient identity.
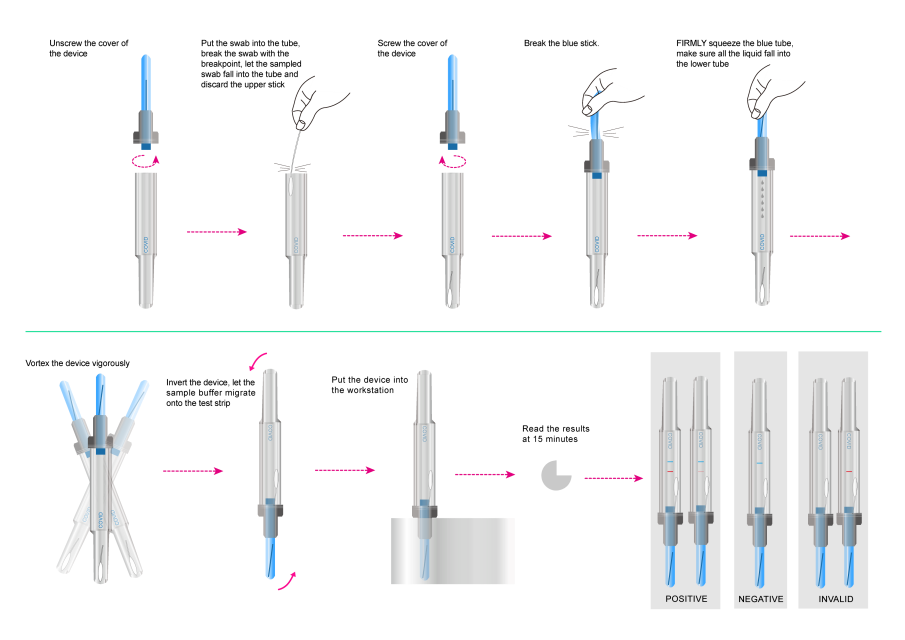
Unscrew the cover of the device.
1. Put the swab into the tube, break the swab with the breakpoint, let the sampled swab fall into the tube and discard the upper stick.
2. Screw the cover of the device.
3. Break the blue stick.
4. FIRMLY squeeze the blue tube, make sure all the liquid fall into the lower tube.
5. Vortex the device vigorously.
6. Invert the device, let the sample buffer migrate onto the test strip.
7. Put the device into the workstation.
8. At the end of 15 minutes read the results. A strong positive sample may show result earlier.
Note: Result after 15 minutes may not be accurate.
LIMITATIONS OF THE TEST
1. The contents of this kit are to be used for the qualitative detection of SARS-CoV-2 antigens from throat swab and nasopharyngeal swab.
2. This test detects both viable (live) and non-viable, SARS-CoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
3. A negative test result may occur if the level of antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly.
4. Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result.
5. Test results must be evaluated in conjunction with other clinical data available to the physician.
6. Positive test results do not rule out co-infections with other pathogens.
7. Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
8. Negative results should be treated as presumptive and confirmed with an FDA authorized molecular assay, if necessary, for clinical management, including infection control.
9. Specimen stability recommendations are based upon stability data from influenza testing and performance may be different with SARS-CoV-2. Users should test specimens as quickly as possible after specimen collection.
10. The sensitivity for RT-PCR assay in diagnosis of COVID-19 is only 50%-80% due to poor sample quality or disease time point at the recoverd phase , etc. SARS-CoV-2 Antigen Rapid Test Device’s sensitivity is theoretically lower because of its Methodology.
Product detail pictures:



Related Product Guide:
To meet the customers' over-expected gratification , we have our robust crew to offer our best over-all support which includes marketing, income, coming up with, production, excellent managing, packing, warehousing and logistics for 2020 China New Design Sars-Cov-2 Antigen - Dual Biosafety System Device for SARS-CoV-2 Antigen Rapid Test – Liming Bio , The product will supply to all over the world, such as: Cairo, Manchester, Egypt, Many kinds of different solutions are available for you to choose, you can do one-stop shopping here. And customized orders are acceptable. Real business is to get win-win situation, if possible, we would like to deliver more support for customers. Welcome all nice buyers communicate details of solutions with us!!
This is a very professional wholesaler, we always come to their company for procurement, good quality and cheap.