News
-

New product launched!
Pet detection product is online. Please contact us if you need it. Nina Wang:sales@limingbio.com Vicky Chen:vickychen@limingbio.comRead more -

The 87th China International Medical Device Expo CMEF successfully concluded
The equipment festival ignites Shencheng! On May 17th, the 87th China International Medical Device (Spring) Expo (CMEF) successfully concluded, attracting a large number of medical device manufacturers, distributors, doctors, resea...Read more -
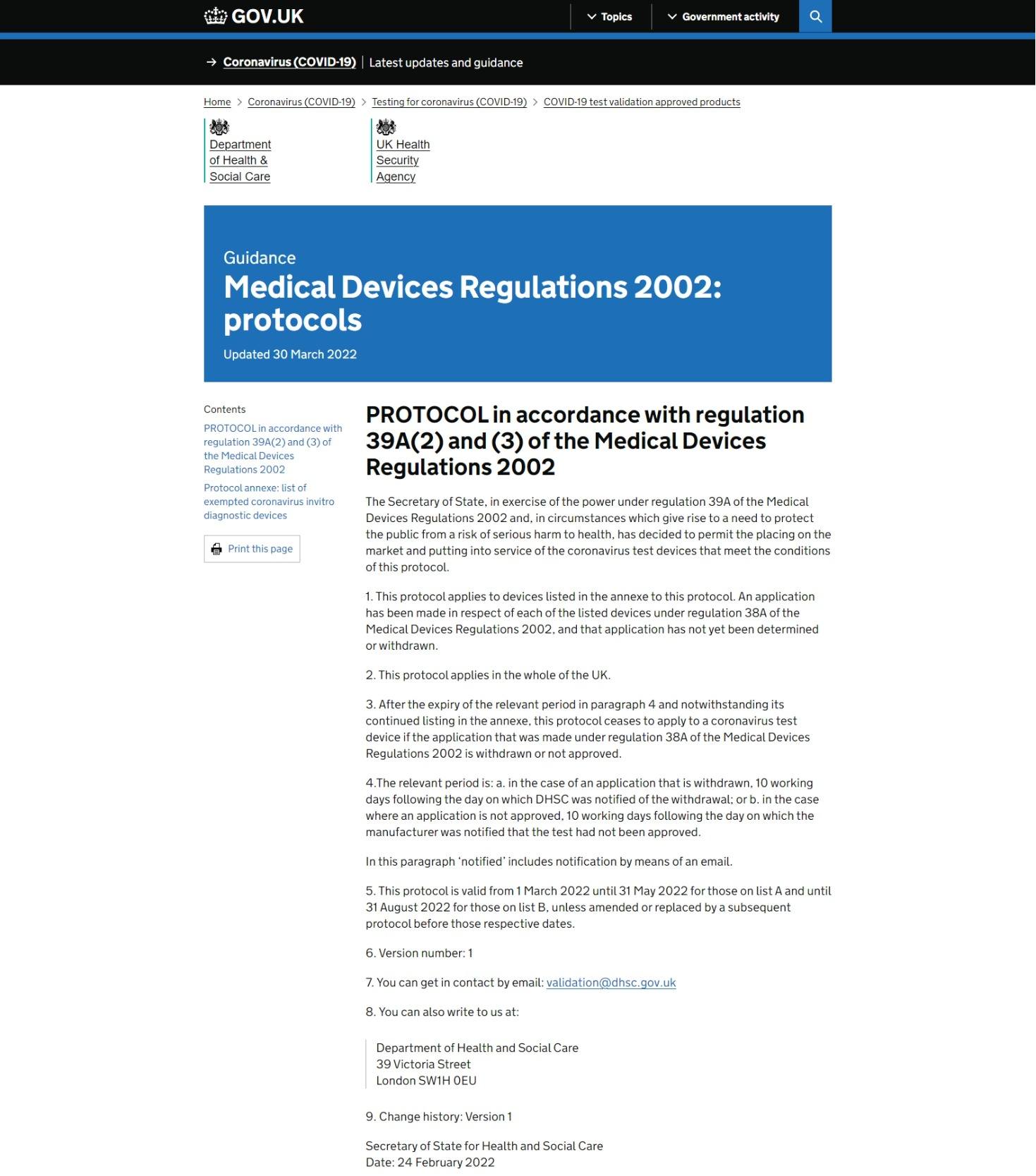
Our products have entered the UK list of exempted coronavirus invitro diagnostic devices!
You can check the list on the UK Department of Health website : https://www.gov.uk/.../medical-devices-regulations-2002... If you need to purchase our products, you can contact us at any time!Read more -
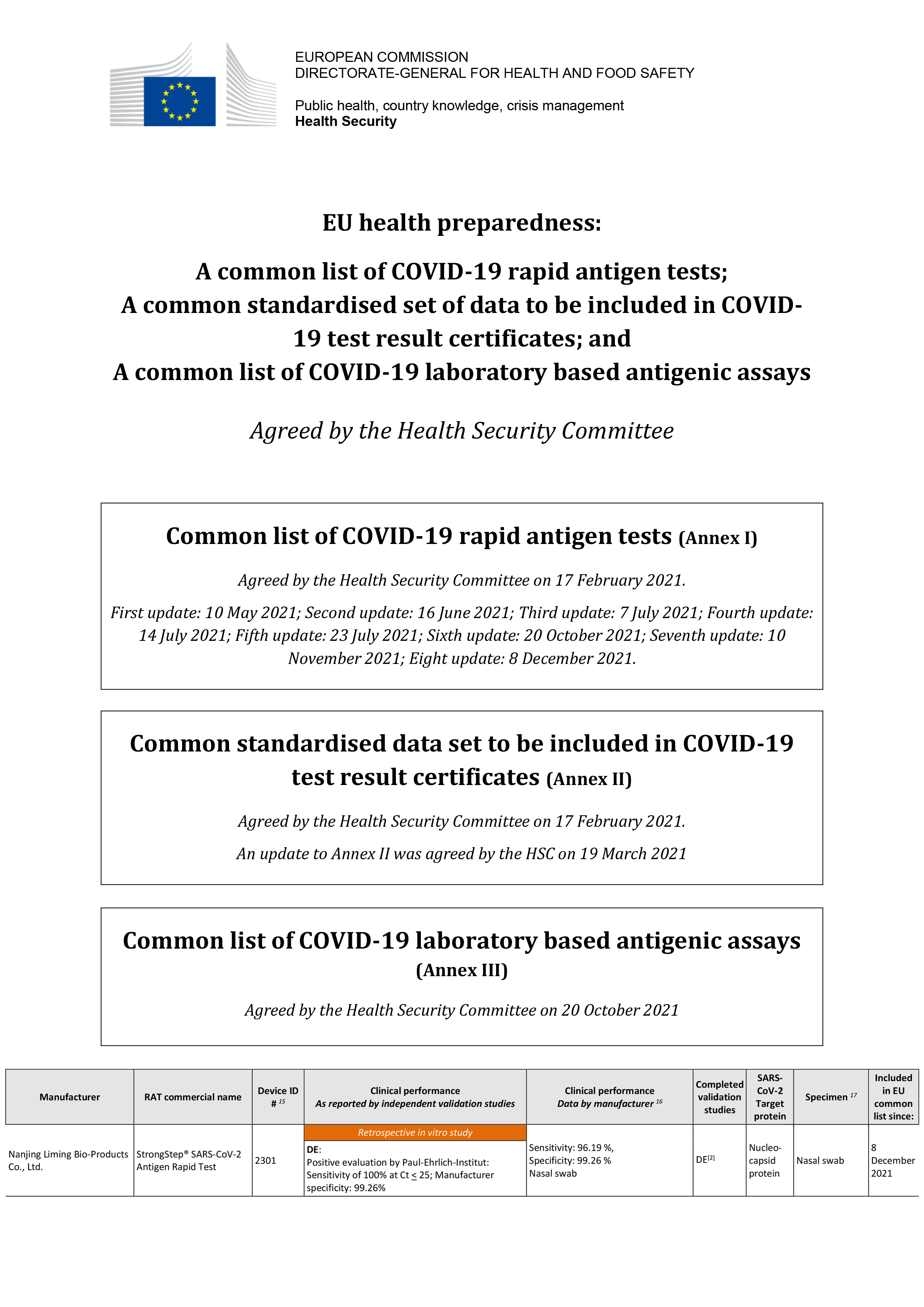
StrongStep® SARS-CoV-2 Antigen Rapid Test Enter the EU common list of hygiene and food safety
StrongStep® SARS-CoV-2 Antigen Rapid Test Enter the EU common list of hygiene and food safety, which is one of the few manufacturers that has 100% sensitivity when the CT value is less than 25%.Read more -

StrongStep® SARS-CoV-2 Antigen Rapid Test included in the FIND evaluation list
StrongStep® SARS-CoV-2 Antigen Rapid Test ncluded in the FIND evaluation list. The Foundation for Innovative New Diagnostics (FIND), is an organization that specializes in evaluating the performance of kits in strategic cooperation with WHO. ...Read more -
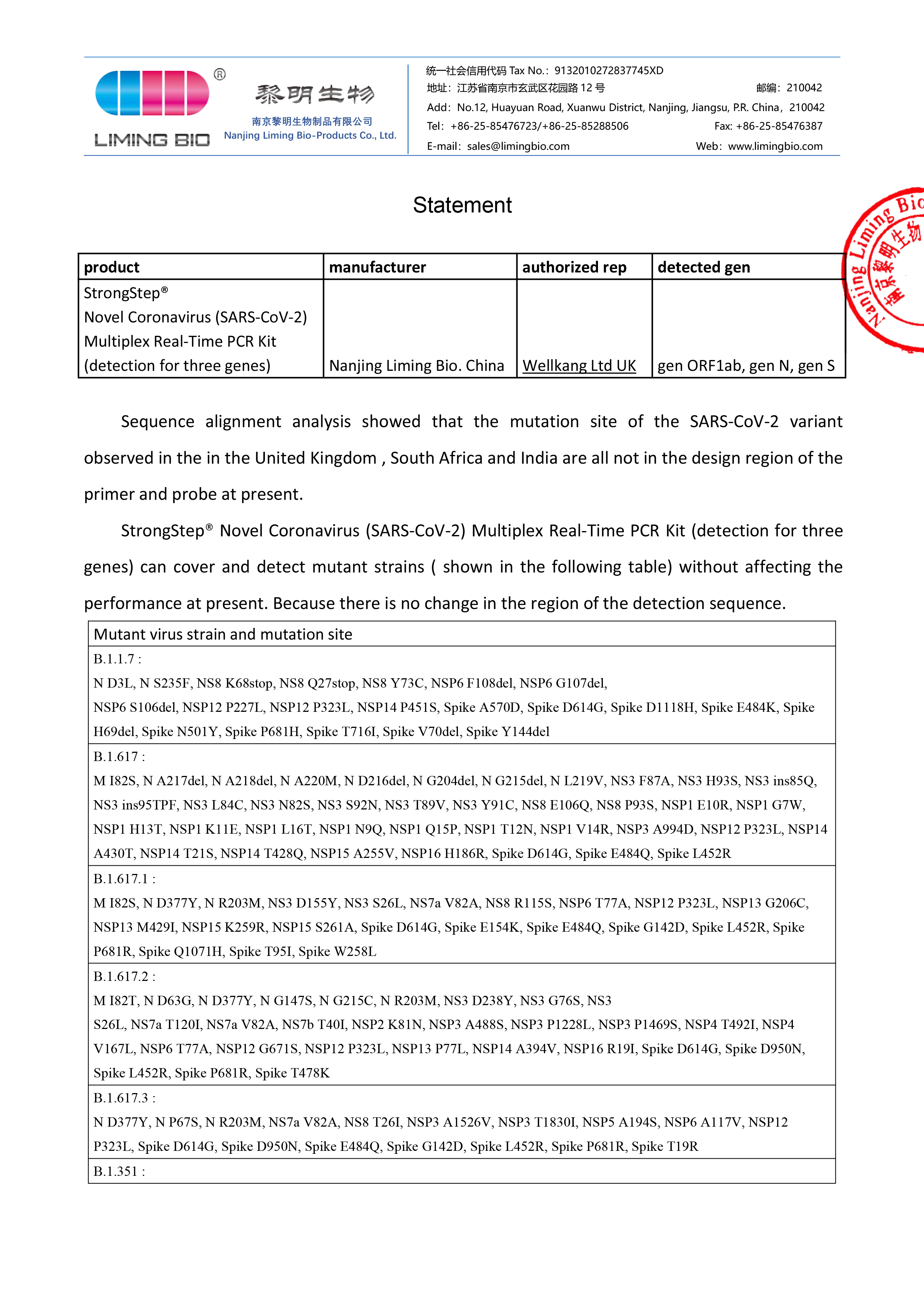
Statement on variant viruses
Sequence alignment analysis showed that the mutation site of the SARS-CoV-2 variant observed in the in the United Kingdom , South Africa and India are all not in the design region of the primer and probe at present. StrongStep® Novel Coronavirus (SARS-CoV-2) Multiplex R...Read more -

In Silico Analysis for StrongStep® SARS-CoV-2 Antigen Rapid Test on Different SARS-CoV-2 Variant
SARS-CoV-2 has now evolved several mutations with serious consequences,some like B.1.1.7,B.1.351,B.1.2,B.1.1.28,B.1.617,Including the omicron mutant strain(B1.1.529) reported in recent days. As an IVD reagent manufacturer, we always pay attention to the developmen...Read more -
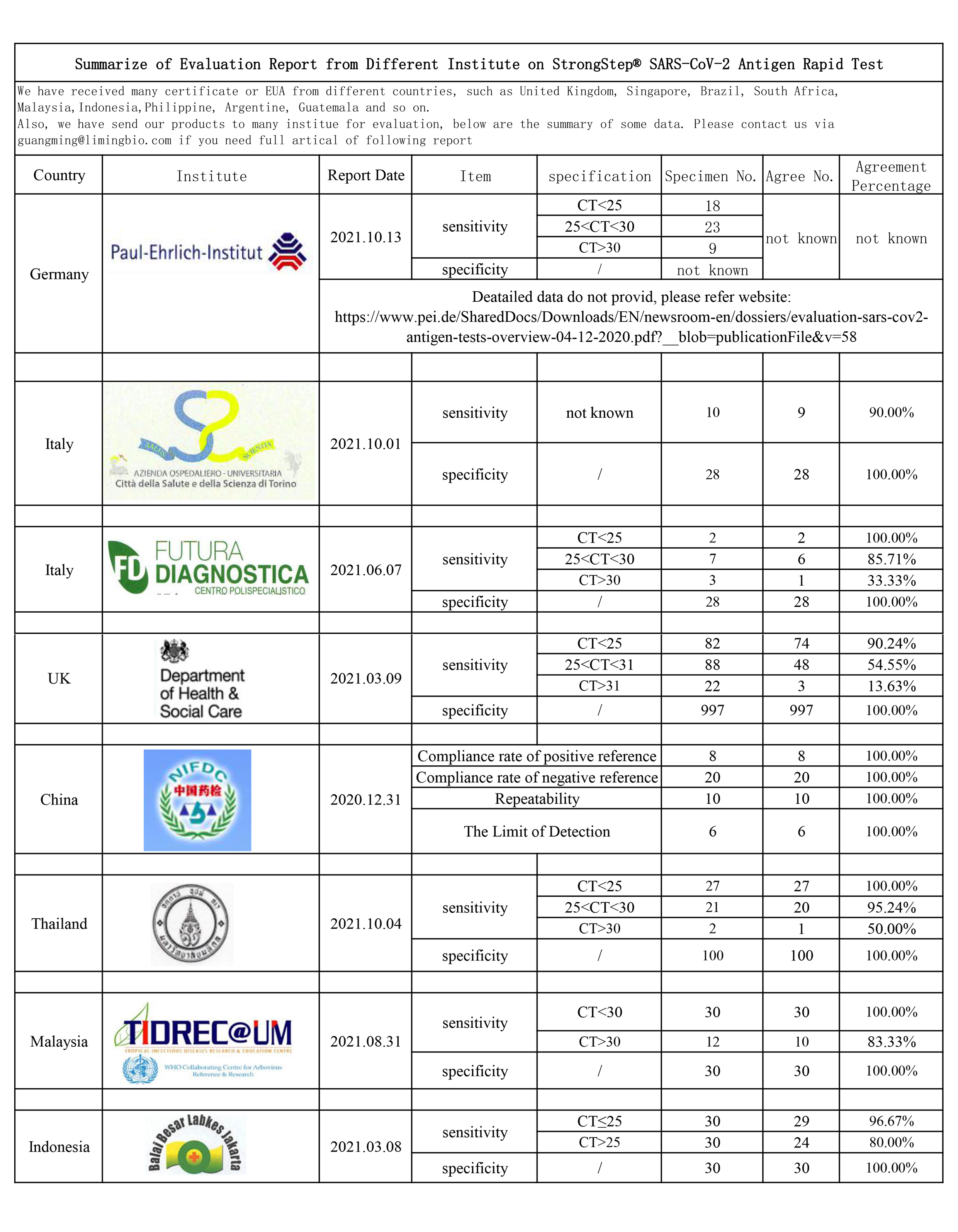
Summarize of Evaluation Report from Different Institute on StrongStep® SARS-CoV-2 Antigen Rapid Test
We have received many certificate or EUA from different countries, such as United Kingdom, Singapore, Brazil, South Africa, Malaysia,Indonesia,Philippine, Argentine, Guatemala and so on. Also, we have send our products to many institue for evaluation, below are the summ...Read more -

The StrongStep SARS-CoV-2 Antigen Rapid Test has successfully obtained the Thailand FDA Certificate!
Recently, the StrongStep® SARS-CoV-2 Antigen Rapid Test produced by Nanjing Liming Bio-products Co. Ltd has successfully obtained the Thailand FDA certificate (registration number T 6400429,T 6400430,T 6400431,T 6400432 ), and now has been approved to enter the Thailand ...Read more -
Nanjing LimingBio’s Novel Coronavirus (SARS-CoV-2) antigen detection reagent “StrongStep® SARS-CoV-2 Antigen Rapid Test” has obtained the performance verification of Paul-Ehrlich-...
Recently, Nanjing LimingBio‘s Novel Coronavirus (SARS-CoV-2) antigen detection reagent "StrongStep® SARS-CoV-2 Antigen Rapid Test" has obtained the performance verification of Paul-Ehrlich-Institut (PEI*) in Germany, this product has been certified by the German Federal ...Read more -
We have obtained import permit from Bangladesh!
G-Tech Solution LtdRead more -

We Obtained South Africa registration certificate
Section 21 Authorisation_V Care Medi Products Pty Ltd_StrongStep® SARS-CoV-2 Antigen Rapid Test_16092021.docxRead more







