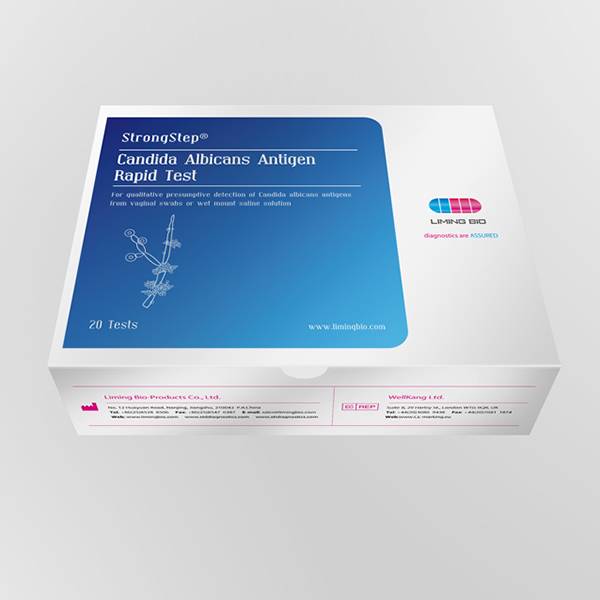
Factory source Candida Albicans Yeast Infection - Candida Albicans Antigen Rapid Test – Liming Bio
Factory source Candida Albicans Yeast Infection - Candida Albicans Antigen Rapid Test – Liming Bio Detail:

INTRODUCTION
Vulvovaginal candidiasis (WC) is thought to be one of the most common causes of vaginal symptoms. Approximately, 75% of women will be diagnosed with Candida at least once during their lifetime. 40-50% of them will suffer recurrent infections and 5% are estimated to develop chronic Candidiasis. Candidiasis is more commonly misdiagnosed than other vaginal infections. Symptoms of WC which include: acute itching, vaginal soreness, irritation, rash on the outer lips of the vagina and genital burning that may increase during urination, are non specific. To obtain an accurate diagnosis, a thorough evaluation is necessary. In women who complain of vaginal symptoms, standard tests should be performed, such as saline and 10% potassium hydroxide microscopy. Microscopy is the mainstay in the diagnosis of WC, yet studies show that, in academic settings, microscopy has a sensitivity of at best 50% and thus will miss a substantial percentage of women with symptomatic WC. To increase the accuracy of diagnosis, yeast cultures have been advocated by some experts as an adjunctive diagnostic test, but these cultures are expensive and underutilized, and They have the further disadvantage that it may take up to a week to get a positive result. Inaccurate diagnosis of Candidiasis may delay treatment and cause more serious lower genital traa diseases. StrongStep9 Candida albicans Antigen Rapid Test is a point-of-care test for qualitative detection of Candida vaginal discharge swabs within 10-20 minutes. It is an important advance in improving the diagnosis of women with WC.
PRECAUTIONS
• For professional in vitro diagnostic use only.
• Do not use after expiration date indicated on the package. Do not use the test if its foil pouch is damaged. Do r>ot reuse tests.
• This kit contains products of animal origin. Certified knowledge of the origin and/or sanitary state of the animals does not totally guarantee the absence of transmissible pathogenic agents. It is therefore, recommended that these products be treated as potentially infectious, and handled observing the usual safety precautions (do not ingest or inhale).
• Avoid cross-contamination of specimens by using a new specimen collection container for each specimen obtained.
• Read the entire procedure carefully prior to performing any tests.
• Do not eat, drink or smoke in the area where the specimens and kits are handled. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow
the standard procedures for proper disposal of specimens. Wear protective clothing such as laboratory coats, disposable gtoves and eye protection when specimens are assayed.
• Do not interchange or mix reagents from different lots. Do not mix solution bottle caps.
• Humidity and temperature can adversely affect results.
• When the assay procedure is completed, dispose the swabs carefully after autoclaving them at 121°C for at least 20 minutes. Alternatively, they can be treated with 0.5% sodium hypochloride (or house-hold bleach) for one hour before disposal. The used testing materials should be discarded in accordance with local, state and/or federal regulations.
• Do not use cytology brushes with pregnant patients.



Candida 1
Candida 2
Candida albicans
Clinical Study_Candida
MSDS for Candida Test
Product detail pictures:

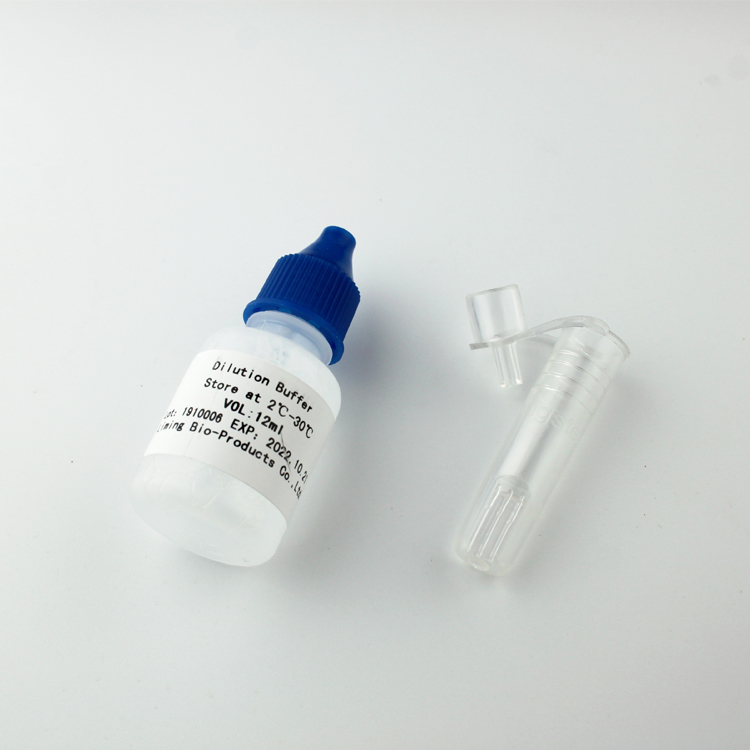

Related Product Guide:
Bear "Customer first, Quality first" in mind, we work closely with our customers and provide them with efficient and professional services for Factory source Candida Albicans Yeast Infection - Candida Albicans Antigen Rapid Test – Liming Bio , The product will supply to all over the world, such as: South Korea, Vietnam, UK, Our company has built stable business relationships with many well-known domestic companies as well as oversea customers. With the goal of providing high quality products to customers at low cots, we've been committed to improving its capacities in research, development, manufacturing and management. We have honored to receive recognition from our customers. Till now we have now passed ISO9001 in 2005 and ISO/TS16949 in 2008. Enterprises of "quality of survival, the credibility of development" for the purpose, sincerely welcome domestic and foreign businessmen to visit to discuss cooperation.
A nice supplier in this industry, after a detail and careful discussion, we reached a consensus agreement. Hope that we cooperate smoothly.