Hot New Products Dual Biosafety System Device For Sars-Cov-2 Antigen Test - SARS-CoV-2 IgM/IgG Antibody Rapid Test – Liming Bio
Hot New Products Dual Biosafety System Device For Sars-Cov-2 Antigen Test - SARS-CoV-2 IgM/IgG Antibody Rapid Test – Liming Bio Detail:
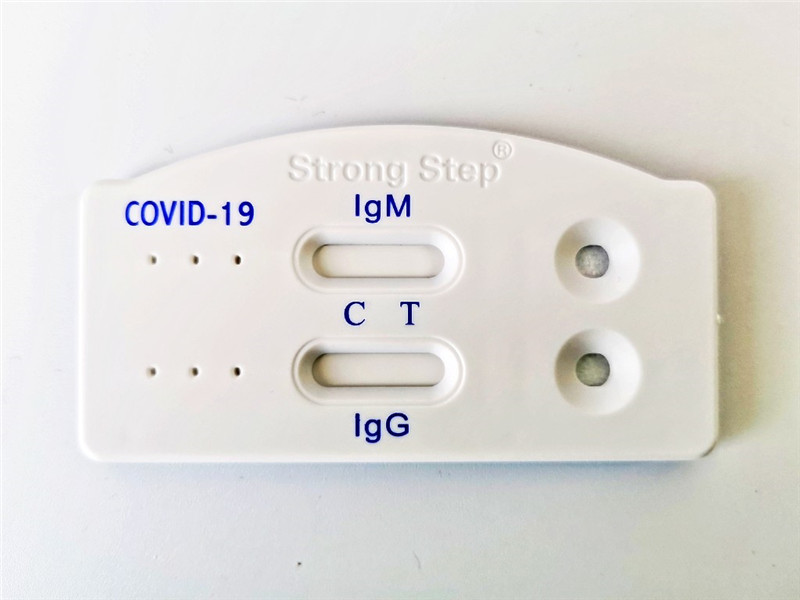
Strongstep® SARS-CoV-2 IgG/IgM Antibody Rapid Test
They can also identify whether they have previously been infected with the SARS-CoV-2 virus and have recovered.This test is only authorized to detect SARS-CoV-2 specific IgM and IgG antibodies.IgG and lgM antibodies to 2019 Novel Coronavirus can be detected with 2-3 weeks after exposure. Negative results do not preclude acute SARS-CoV-2 infection. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. lgG remains positive, but the antibody level drops overtime. It is not applicable to any other viruses or pathogens, and the results should not be used to diagnose or rule out SARS-CoV infection or inform the status of infection.
If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
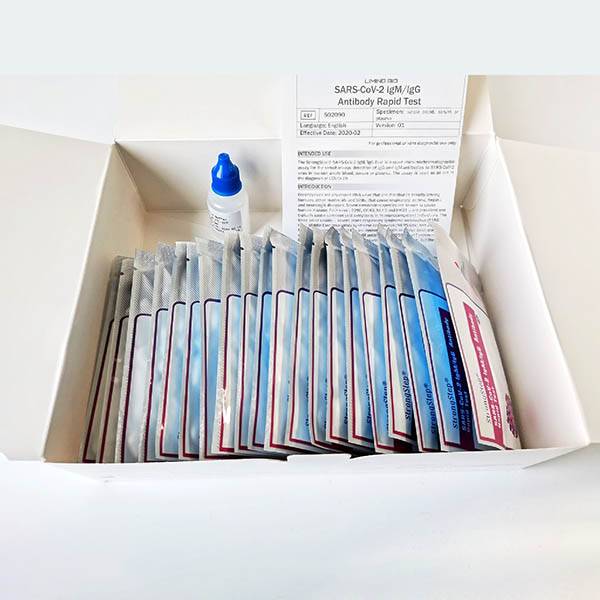
INTENDED USE
TheStrongStep® SARS-CoV-2 IgM/IgG Test is a rapid immuno-chromatographic assay for the simultaneous detection of IgM and IgG antibodies to SARS-CoV-2 virus in human whole blood, serum or plasma. The assay is used as an aid in the diagnosis of COVID-19.
INTRODUCTION
Coronavirus is enveloped RNA virus distributed broadly among humans, other mammals and birds, which cause respiratory, enteric, hepatic and neurologic diseases. Seven coronavirus species are known to cause human disease. Four virus strains – 229E, OC43, NL63 and HKU1 – are prevalent and typically cause common cold symptoms in immuno-competent individuals. The three other strains – severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and 2019 Novel Coronavirus (COVID-19) – are zoonotic in origin and have been linked to sometimes fatal illness, Coronavirus is zoonotic, which meaning they can be transmitted between animals and people. Common signs of infection include respirator symptoms, fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure and even death. IgM and IgG antibodies to 2019 Novel Coronavirus can be detected with 1-2 weeks after exposure. IgG remains positive, but the antibody level drops overtime.
PRINCIPLE
TheStrongStep®SARS-CoV-2 IgM/IgG Test utilizes the principle of Immuno-chromatography. Each device contain two strips, where SARS-CoV-2 specific recombinant antigen immobilized on the nitrocellulose membrane within test window of device. Mouse anti-human IgM and anti-human IgG antibodies conjugated with colored latex beads are immobilized on the conjugate pad of the two strips respectively. As the test sample flows through the membrane within the test device, the colored mouse anti-human IgM and anti-human IgG antibodies form latex conjugate complexes with human antibodies (IgM and/or IgG). This complex moves further on the membrane to the test region where it is captured by SARS-CoV-2 specific recombinant antigen. If SARS-CoV-2 virus IgG/IgM antibodies present in the sample, which is leading to formation of a colored band and it indicates a positive test results. Absence of this colored band within the test window indicates a negative test result. This complex moves further on the membrane to the control region where it is captured by goat anti-mouse antibody and forms red control line which is a built-in control line that will always appear in the test window when the test is performed properly, regardless of the presence or absence of anti-SARS-CoV-2 virus antibodies in the specimen.
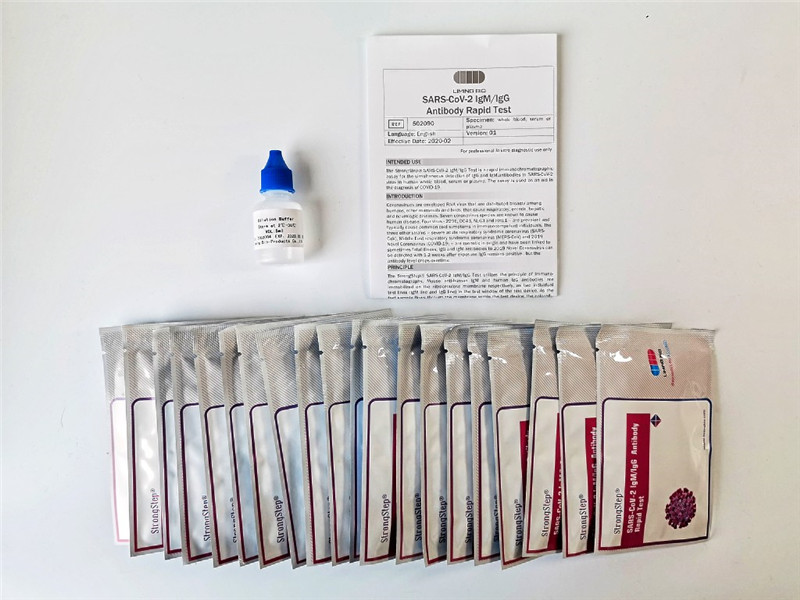
KIT COMPONENTS
1. StrongStep® SARS-CoV-2 IgM/IgG Test Card in foil pouch
2. Sample Buffer
3. Instructions for Use
MATERIALS REQUIRED BUT NOT PROVIDED
1. Sepcimen collection container
2. 1-20μL Pipetter
3. Timer
The test is limited in the US to distribution to laboratories certified by CLIA to perform high complexity testing.
This test has not been reviewed by the FDA.
Negative results do not preclude acute SARS-CoV-2 infection.
If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
Results from antibody testing should not be used to diagnose or exclude acute SARS-CoV-2 infection.
Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
Product detail pictures:





Related Product Guide:
Our enterprise since its inception, constantly regards product good quality as organization life, constantly improve production technology, strengthen merchandise high quality and continuously strengthen enterprise total good quality administration, in strict accordance with all the national standard ISO 9001:2000 for Hot New Products Dual Biosafety System Device For Sars-Cov-2 Antigen Test - SARS-CoV-2 IgM/IgG Antibody Rapid Test – Liming Bio , The product will supply to all over the world, such as: UAE, Mombasa, Manila, During in 11 years, We've participated in more than 20 exhibitions, obtains the highest praise from each customer. Our company has been devoting that "customer first" and committed to helping customers expand their business, so that they become the Big Boss !
The goods are very perfect and the company sales manager is warmful, we will come to this company to purchase next time.