Human Diagnostics
-
Monkeypox Virus Generic qPCR Assay
REF 510080 Specification 96 Test/box Detection principle Nucleic acid Specimens Blisters fluid, pustules fluid, lesionized skin rash, whole blood or serum, throat swab Intended Use This kit is used for in vitro qualitative detection of monkeypox virus nucleic acids in vesicle fluid, pustule fluid, lesion skin rash, whole blood or serum, throat swabs and other suspicious samples from suspected monkeypox virus-infected individuals, and it can also be used for typing (West African type and Congo type). -
18 types of high risk human papillomavirus nucleicacid detection and 16/18 Typing Kit
REF 510070 Specification 96 Test/box Detection principle Nucleic acid Specimens female cervical exhumated epithelial cells Intended Use This kit is mainly used for qualitative detection of 18 high-risk human papillomavirus (HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) DNA in female cervical exhumated epithelial cells in vitro, and can specifically identify HPV16 and HPV18. -
Influenza A and B virus,respiratory syncytial virus nucleic acid detection kit (lyophilized type) (PCR-fluorescence probe method)
REF 510030 Specification 96 Test/box Detection principle Nucleic acid Specimens Oral pharyngeal swab Intended Use This kit is used for qualitative detection of influenza A virus (IFVA), influenza B virus (IFVB), and respiratory syncytial virus (RSV) RNA in human throat swab samples. -
Influenza A/B Multiplex RT-qPCR Kit
REF 510020 Specification 96 Test/box Detection principle Nucleic acid Specimens nasopharyngeal swabs and oropharyngeal swabs Intended Use This kit is intended for simultaneous qualitative detection and differentiation of nucleic acid from influenza A and influenza B in nasopharyngeal swabs and oropharyngeal swabs. -

SARS-CoV-2 IgM/IgG Antibody Rapid Test
REF 502090 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens Whole Blood / Serum / Plasma Intended Use This is a rapid immuno-chromatographic assay for the simultaneous detection of IgM and IgG antibodies to SARS-CoV-2 virus in human whole blood, serum or plasma. The test is limited in the US to distribution to laboratories certified by CLIA to perform high complexity testing.
This test has not been reviewed by the FDA.
Negative results do not preclude acute SARS-CoV-2 infection.
Results from antibody testing should not be used to diagnose or exclude acute SARS-CoV-2 infection.
Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
-

SARS-CoV-2 Antigen Rapid Test(nasal)
REF 500200 Specification 1 Tests/Box ;5 Tests/box ; 20 Tests/box Detection principle Immunochromatographic assay Specimens Anterior nasal swab Intended Use StrongStep® SARS-CoV-2 Antigen Rapid Test Cassette employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human anterior nasal swab specimen. This testis single use only and intended for self-testing. It is recommanded to use this test within 5 days of symptom onset. It is supported by the clinical performance assessment. -

Dual Biosafety System Device for SARS-CoV-2 Antigen Rapid Test
REF 500210 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens Nasal / Oropharyngeal swab Intended Use This is a rapid immunochromatographic assay for the detection of SARS-CoV-2 virus Nucleocapsid Protein antigen in human Nasal /Oropharyngeal swab collected from individuals who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms. The assay is used as an aid in the diagnosis of COVID-19. -
System Device for SARS-CoV-2 & Influenza A/B Combo Antigen Rapid Test
REF 500220 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens Nasal / Oropharyngeal swab Intended Use This is a rapid immunochromatographic assay for the detection of SARS-CoV-2 virus Nucleocapsid Protein antigen in human Nasal/Oropharyngeal swab collected from individuals who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms. The assay is used as an aid in the diagnosis of COVID-19. -
1人份抗原卡实物图唾液版1_00_副本-300x216.png)
SARS-CoV-2 Antigen Rapid Test for Saliva
REF 500230 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens SalivaIntended Use This is a rapid immunochromatographic assay for the detection of SARS-CoV-2 virus Nucleocapsid Protein antigen in human Saliva swab collected from individuals who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms. The assay is used as an aid in the diagnosis of COVID-19. -
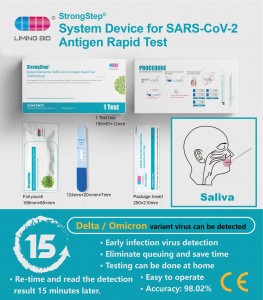
StrongStep System Device for SARS-CoV-2 Antigen Rapid Test
REF 500210 Specification 1 Test/box Detection principle Immunochromatographic assay Specimens SalivaIntended Use StrongStep System Device for SARS-CoV-2Antigen Rapid Test employs immunochromatography technobgy to detect the SARS-CoV-2 nucleocapsid antigen in human saliva. This test is single use only and intended for self-testing. It is recommended to use this test within 7 days of symptom onset. lt is supported by the dinical performance assessment. -

Dermatophytosis Diagnostic kit
REF 500280 Specification 20 Test/box Detection principle Immunochromatographic assay Specimens Swab/ Nails/ Scurf/ Hair Intended Use The StrongStep®Dermatophytosis Diagnostic kit is a rapid visual immunoassay for the qualitative presumptive detection of α-1, 6 mannose in fungi belonging to dermatophytes. This kit is intended to be used as an aid in the diagnosis of Dermatophytosis. -

Human Fecal Occult Blood Rapid Test
REF 501060 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens Cervical/urethra swab Intended Use The StrongStep® FOB Rapid Test Device (Feces) is a rapid visual immunoassay for the qualitative presumptive detection of human hemoglobin in human fecal specimens.







