Manufacturer for Sars-Cov-2 Antigen Rapid Test - SARS-CoV-2 Antigen Rapid Test(Professional Use) – Liming Bio
Manufacturer for Sars-Cov-2 Antigen Rapid Test - SARS-CoV-2 Antigen Rapid Test(Professional Use) – Liming Bio Detail:
INTENDED USE
The StrongStep® SARS-CoV-2 Antigen Rapid Test is is a rapid immunochromatographic assay assayfor the detection of SARS-CoV-2 virus Nucleocapsid Protein antigen in human Nasalv collected fromindividuals who are Asymptomatic or, Symptomatic of being infected with COVID-19 within the first fivedays of the onset of symptoms. The assay is used as an aid in the diagnosis of COVID-19. It is designedto be used for infection screening and auxiliary diagnosis in Symptomatic and Asymptomatic people.
INTRODUCTION
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found ina few cases.
PRINCIPLE
The StrongStep® SARS-CoV-2 Antigen Test employs immunochromatographic test. Latex conjugated antibodies (Latex-Ab) corresponding to SARS-CoV-2 are dry-immobilized at the end of nitrocellulose membrane strip. SARS-CoV-2 antibodies are bond at the Test Zone (T) and Biotin-BSA is bond at the Control Zone (C).When the sample is added, it migrates by capillary diffusion rehydrating the latex conjugate. If present in sample, SARS-CoV-2 antigens will bind with the conjugated antibodies forming particles. These particles will continue to migrate along the strip until the Test Zone (T) where they are captured by SARS-CoV-2 antib odies generating a visible red line. If there are no SARS-CoV-2 antigens in sample, no red line is formed in the Test Zone (T). The streptavidin conjugate will continue to migrate alone until it is captured in the Control Zone(C) by the Biotin-BSA aggregating in a blue line, which indicates the validity of the test.
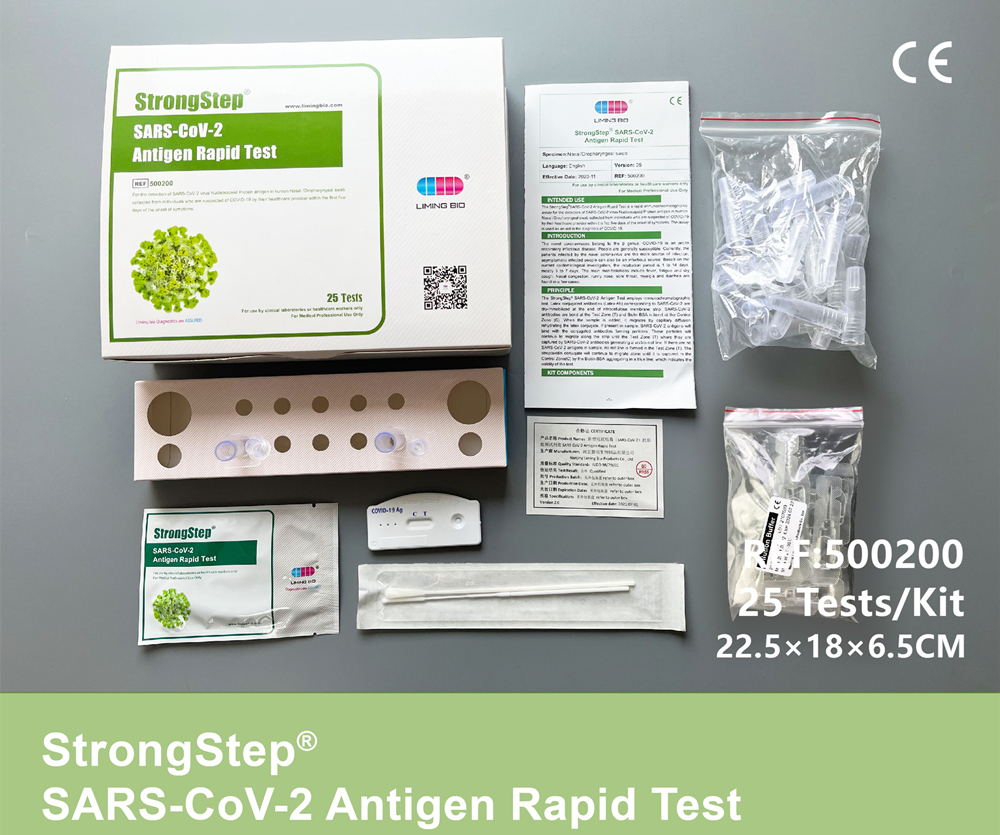
KIT COMPONENTS
| 25 sealed foil pouch packed test devices | Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding regions. |
| 25 Extraction tubes with pre-filled dilution buffer |
0.1 M Phosphate buffered saline (PBS) and 0.02% sodium azide. |
| 25 Packs of swab | For specimen collection. |
| 1 Workstation | Place for holding buffer vials and tubes. |
| 1 Package insert | For operation instruction. |
KIT COMPONENTS
| Timer | For timing use. |
| Any necessary personal protective equipment |
PRECAUTIONS
• This kit is for IN VITRO diagnostic use only.
• This kit is for medical professional use only.
• Read the instructions carefully before performing the test.
• This product does not contain any human source materials.
• Do not use kit contents after the expiration date.
• Handle all specimens as potentially infectious.
• Follow standard Lab procedure and biosafety guidelines for handling and disposal of potentially infective material. When the assay procedure is complete, dispose specimens after autoclaving them at 121℃ for at least 20 minutes. Alternatively, they can be treated with 0.5% Sodium Hypochlorite four hours before disposal.
• Do not pipette reagent by mouth and no smoking or eating while performing assays.
• Wear gloves during the whole procedure.
• It is recommend to use Liming Bio’s System Device For Rapid Detection of SARS-CoV-2 Antigen (Cat # 500210) to protect the operator and environment.
STORAGE AND STABILITY
The sealed pouches in the test kit may be stored between 2-30℃ for the duration of the shelf life as indicated on the pouch.
SPECIMEN COLLECTION AND STORAGE
Nasal Swab Sample:
• Insert one swab into one nostril of the patient. The swab tip should be inserted up to 2.5 cm (1 inch) from the edge of the nostril. Roll the swab 5 times along the mucosa inside the nostril to ensure that both mucus and cells are collected.
• Use the same swab, repeat this process for the other nostril to ensure that an adequate sample is collected from both nasal cavities.
Use the swab supplied in the kit, alternative swabs may adversely affect test performance, uses should validate their swab before use it. It is recommended that specimens be processed as soon as possible after collection. Specimens can be held in container up to 1 hour at room temperature (15°C to 30°C), or up to 24 hours when refrigerated (2°C to 8°C) before processing.
PROCEDURE
Bring test devices, specimens, buffer and/or controls to room temperature (15-30°C) before use.
• For pre-filled buffer, remove the seal from the vial containing the liqutd.
• Put the specimen swab into the tube. Vigorously mix the solution by rotating the swab forcefully against the side of the tube for least 15 times (while submerged). Best results are obtained when the specimen is vigorously mixed in the solution.
• Allow the swab to soak in the Extraction Buffer for one minute prior to the next Step
• Squeeze out as much liquid as possible from the swab by pinching the side of the flexible extraction tube as the swab is removed. At least 1/2 of the sample buffer solution must remain in the tube for adequate capillary migration to occur. Put the cap onto the extracted tube.
• Discard the swab in a suitable biohazardous waste container.
• Cover the cap.
• Mix the solution by squeeze the specimen forcefully against the side of the tube for at least ten times
(while submerged). Best results are obtained when the specimen is mixed in the solution. Allow the specimen to soak in the Dilution Buffer for one minute prior to the next step.
• The specimens extracted can retain at room temperature for 30 minutes without affecting the result of the test.
• Remove the test device from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. To obtain a best result, the assay should be performed within 30 minutes.
• Add 3 drops (approximately 100 µL) of extracted sample from the Extraction Tube to the round sample well on the test device.
• Avoid trapping air bubbles in the sample well (S), and do not drop any solution in observation window. As the test begins to work, you will see color move across the membrane.
• Wait for the colored band(s) to appear. The result should be read by visual at 15 minutes. Do not interpret the result after 30 minutes.
Discard used Extraction Tubes and Test Devices in suitable biohazardous waste container.
Product detail pictures:

Related Product Guide:
abide by the contract", conforms to the market requirement, joins during the market competition by its superior quality also as provides extra comprehensive and exceptional service for consumers to let them turn into significant winner. The pursue of the business, is definitely the clients' gratification for Manufacturer for Sars-Cov-2 Antigen Rapid Test - SARS-CoV-2 Antigen Rapid Test(Professional Use) – Liming Bio , The product will supply to all over the world, such as: Nicaragua, Norwegian, Greek, We supply professional service, prompt reply, timely delivery, excellent quality and best price to our customers. Satisfaction and good credit to every customer is our priority. We focus on every detail of order processing for customers till they have received safe and sound products with good logistics service and economical cost. Depending on this, our products are sold very well in the countries in Africa, the Mid-East and Southeast Asia.
The product manager is a very hot and professional person, we have a pleasant conversation, and finally we reached a consensus agreement.