OEM manufacturer Real Time Pcr Primer Design - FOB Rapid Test – Liming Bio
OEM manufacturer Real Time Pcr Primer Design - FOB Rapid Test – Liming Bio Detail:
NTENDED USE
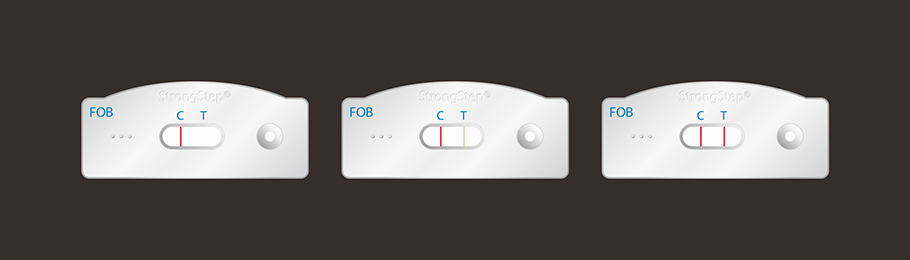
The StrongStep® FOB Rapid Test Strip (Feces) is a rapid visual immunoassay for the qualitative presumptive detection of human hemoglobin in human fecal specimens. This kit is intended to be used as an aid in the diagnosis of lower gastrointestinal (g.i.) pathologies.
INTRODUCTION
Colorectal cancer is one of the most commonly diagnosed cancers and a leading cause of cancer death in the United States. Screening for colorectal cancer probably increases the cancer detection at an early stage, therefore reduces the mortality.
Earlier commercially available FOB tests utilized the guaiac test, which requires special dietary restriction to minimize false positive and false negative results. The FOB Rapid Test Strip (Feces) are especially designed to detect human hemoglobin in fecal samples using Immunochemical methods, which improved specificity for the detection of lower gastrointestinal. disorders, including colorectal cancers and adenomas.
PRINCIPLE
The FOB Rapid Test Strip (Feces) has been designed to detect human hemoglobin through visual interpretation of color development in the internal strip. The membrane was immobilized with anti-human hemoglobin antibodies on the test region. During the test, the specimen is allowed to react with colored anti-human hemoglobin antibodies colloidal gold conjugates, which were precoated on the sample pad of the test. The mixture then moves on the membrane by a capillary action, and interact with reagents on the membrane. If there were enough human hemoglobin in specimens, a colored band will form at the test region of the membrane. Presence of this colored band indicates a positive result, while its absence indicates a negative result. Appearance of a colored band at the control region serves as a procedural control. This indicates that proper volume of specimen has been added and membrane wicking has occurred.
PRECAUTIONS
■ For professional in vitro diagnostic use only.
■ Do not use after the expiration date indicated on the package. Do not use the test if the foil pouch is damaged. Do not reuse tests.
■ This kit contains products of animal origin. Certified knowledge of the origin and/or sanitary state of the animals does not completely guarantee the absence of transmissible pathogenic agents. It is therefore, recommended that these products be treated as potentially infectious, and handled by observing usual safety precautions (e.g., do not ingest or inhale).
■ Avoid cross-contamination of specimens by using a new specimen collection container for each specimen obtained.
■ Read the entire procedure carefully prior to testing.
■ Do not eat, drink or smoke in any area where specimens and kits are handled. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow standard procedures for proper disposal of specimens. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
■ The specimen dilution buffer contains sodium azide, which may react with lead or copper plumbing to form potentially explosive metal azides. When disposing of specimen dilution buffer or extracted samples, always flush with copious quantities of water to prevent azide buildup.
■ Do not interchange or mix reagents from different lots.
■ Humidity and temperature can adversely affect results.
■ Used testing materials should be discarded according to local regulations.
Product detail pictures:
Related Product Guide:
Quality First,and Customer Supreme is our guideline to provide the best service to our customers.Nowadays, we are trying our best to become one of the best exporters in our field to meet customers more need for OEM manufacturer Real Time Pcr Primer Design - FOB Rapid Test – Liming Bio , The product will supply to all over the world, such as: Bandung, Vietnam, Canada, Item have passed by means of the national qualified certification and been well received in our main industry. Our expert engineering team will often be ready to serve you for consultation and feedback. We have been able to also deliver you with cost-free samples to meet your specs. Ideal efforts will probably be produced to deliver you the most beneficial service and solutions. Should really you be interested in our company and solutions, please make contact with us by sending us emails or call us straight away. To be able to know our solutions and enterprise. ar more, you'll be able to come to our factory to see it. We will constantly welcome guests from all over the world to our firm. o build business enterprise. elations with us. You should feel absolutely free to speak to us for organization. nd we believe we are going to share the best trading practical experience with all our merchants.
The factory has advanced equipment, experienced staffs and good management level, so product quality had assurance, this cooperation is very relaxed and happy!